Bridge the gap between agile and compliance
Introducing an agile framework specialized for FDA-regulated software development
What are non-agile processes costing your company?

- Software releases that are either late or really late?
- Losing the race-to-market against your competition?
- Feeling stuck using old technology?
You need an agile framework specialized for regulated software development.
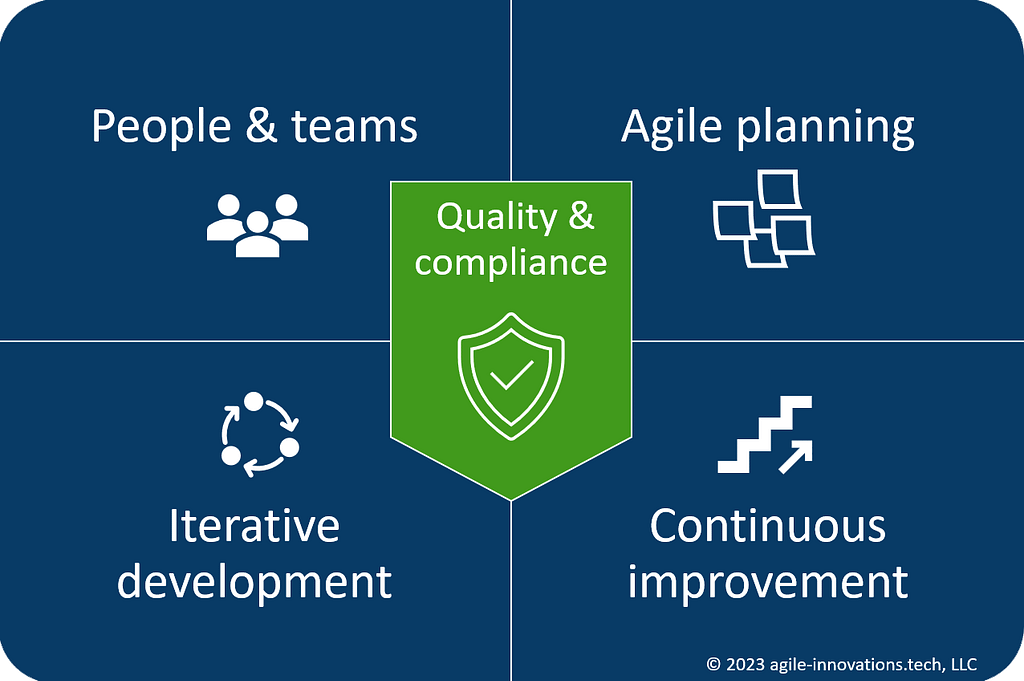
People & teams: Foster self-organizing teams with clearly defined roles, training, and open communications.
Agile planning: Promote customer-centric development with frequent releases of high-value features.
Iterative development: Deliver ready-to-ship software through timeboxed iterations, integrated testing, and process automation.
Continuous improvement: Drive ongoing improvement through retrospective reviews, feedback checkpoints, and metrics/KPIs.
Quality & compliance: Maintain compliance through integrated risk management and audit-ready documentation.
You can bridge the gap between agile and compliance.

Aligning agile software development with industry regulations can be confusing. At first glance, the two worlds couldn’t seem further apart.
And it certainly doesn’t help that the Agile Manifesto seems to shun processes and documentation. 1
How are we supposed to sell that to the guy down in QA? The first time I tried–nearly 15 years ago–I was almost run out of the room!
But first impressions are often wrong. Agile development can be easily aligned with industry regulations.2 My old team eventually figured this out…the hard way. But you can do it faster.
Throughout my career, I’ve worked with dozens of companies–including 11 of the largest life science companies in the world–to modernize their SDLC processes. And I want to show you how to do the same.
1 It actually doesn’t…that’s just a common misconception.
2 FDA agrees and has recognized AAMI’s guidance on agile development as a complete standard.
Which program is right for you?
Agile Bootstrapper SM
A 90-day program for new teams that want to get started on the right foot. Use my turnkey processes to get up and running as fast as possible while ensuring you’ll be compliant and audit-ready.
Agile Pilot SM
A 120-day program for established teams that want to transition from traditional to agile processes. Use my framework to assess your current agility level and create a personalized path to a successful agile transformation.
Agile Accelerator SM
A 180-day program for already-agile teams that want to improve their performance. Use my framework to create more predictable release schedules, higher software quality, and enhanced audit readiness.
Your path to success:
1. Schedule a call
Schedule a commitment-free, two-way interview. Find out if we’re a good fit and which program is right for you.
2. Create an action plan
We’ll work together to create a personalized action plan that leverages my framework to achieve your business goals.
3. Implement & win
I’ll be there every step of the way as you put the plan into action.
An agile framework specialized for FDA-regulated software development
Most life science companies struggle to align agile practices with industry regulations. My agile development framework is FDA-compliant and based on proven best practices so that you can stabilize release schedules, improve software quality, and reduce time to market.
The framework consists of the four (4) pillars of agile software development: People and Teams, Agile Planning, Iterative Development, and Continuous Improvement. At the center of the four pillars is a fifth pillar unique to FDA-regulated industries: Quality and Compliance.

The 5 pillars of agile software development
Each of the pillars contains key practice areas for effective agile software development:
1. People & Teams
Clearly defined roles, committed stakeholders, and open communications.
- Team roles: Ensure your team has all the essential roles for effective agile development: not just developers and testers, but also a product owner, facilitator, executive sponsor, and quality assurance. Through clear role definitions, your team becomes more cohesive and efficient.
- Communication: Identify key stakeholders and engage them early in the process. Implement agile ceremonies (kickoffs, standups, demos, retrospectives, etc.) and other communication systems to ensure tight feedback loops and efficient collaboration within the team and with customers and other stakeholders.
- Training: Ensure everyone understands the benefits and principles of agile software development. Train team members on the responsibilities of their assigned roles. Ensure stakeholders are committed to the process and are willing to be engaged.
2. Agile Planning
Embracing change and prioritizing customer value.
- Requirements analysis: Use just-in-time elaboration to document just the right level of detail at just the right time. Tighter feedback loops with customers and stakeholders support rapid refinement and higher customer satisfaction.
- Software development planning: Emergent design ensures software architecture is lean and provides maximum customer value.
- Release planning: Routine backlog grooming continuously prioritizes items with the highest customer value first. Agile estimation techniques lead to more realistic (and accurate) release schedules. Incremental release planning ensures customer value is delivered early and often, and the development team is never caught empty-handed on release day.
3. Iterative Development
Build…test…demo…repeat
- Agile development: Implement functionality in timeboxed iterations. Use the “Done is done” strategy to ensure the software is always in a near ready-to-ship state.
- Agile testing: Verification and validation (V&V) are conducted continuously throughout development rather than waiting until the end. Testing occurs as part of each backlog item, after each development iteration, and before each release.
- Process automation: Continuous integration and continuous delivery (CI/CD) automate as much of the development process as possible. Automated regression testing runs against every software change. Automated deployments save time and reduce human error.
4. Continuous Improvement
Improving performance by analyzing, learning, and adapting.
- Retrospective reviews: Conduct a retrospective review (or an after-action review) after each development iteration and after each release. Reflecting on past performance drives future performance.
- Feedback loops: Schedule frequent checkpoints with customers and other stakeholders (including auditors). Monitor the performance and real-world usage of the software.
- Metrics and KPIs: Identify, track, and review key performance and quality-related metrics. Use the metrics to identify and address process problems.
5. Quality & Compliance
Ensuring safety, security, and audit readiness.
- Risk management: Incorporate risk management into every stage of development. Leverage agile development’s shorter feedback loops to identify safety and security risks as early as possible.
- Documentation: Maintain all the documentation and records expected by regulatory auditors. Use agile development’s focus on customer (stakeholder) value to avoid producing unnecessary documentation. (Auditors are stakeholders, too!) Use the “Done is done” strategy to maintain consistent documentation throughout, saving effort at release time.
- Process auditing: Ensure audit readiness through routine internal auditing.
Agile Coaching
My coaching begins by evaluating your team’s agile maturity against the framework described above. Based on this assessment, we’ll identify the most valuable areas for improvement. Together, we’ll set practical goals and create an action plan. Then, you’ll put the plan into action, and I’ll be there every step of the way to help you along.
And, of course, we’ll use the principles of agile planning and execution to stay on track!
Common myths about agile development in life sciences
| Myth | Reality |
|---|---|
| Agile development is not compliant with regulatory requirements. | Agile development processes can be mapped to regulatory standards and requirements. |
| Agile development does not allow for adequate testing and validation. | Agile development emphasizes the importance of continuous testing throughout the entire development process, rather than waiting until the end to test everything at once. |
| Agile development does not allow for documentation and traceability. | By embracing Agile’s “Done is Done” mindset, you can ensure documentation and traceability are consistent after each new feature or modification, rather than waiting to find gaps during final release activities. |
| Agile development does not work for safety-critical systems. | Agile development promotes early and continuous feedback, which can help to identify and address potential safety issues more quickly than traditional development approaches. |
| Agile development is too chaotic and unstructured for regulated industries. | The key to making agile development work for regulated industries is to establish clear processes and guidelines that prioritize safety, quality, and compliance. |
| FDA does not accept agile methodologies. | FDA has accepted AAMI TIR45 (Guidance on the use of AGILE practices in the development of medical device software) as a complete standard for software development. |